
SL Paper 1
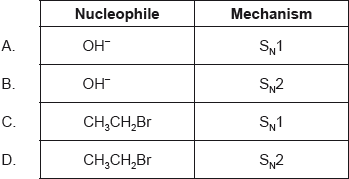
Which combination best describes the substitution reaction between bromoethane and dilute aqueous sodium hydroxide?
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}} + {\text{O}}{{\text{H}}^ - } \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}} + {\text{B}}{{\text{r}}^ - }\]
Markscheme
B
Examiners report
In organic reaction mechanisms, what does a curly arrow represent?
A. The movement of a pair of electrons towards a nucleophile
B. The movement of a pair of electrons towards a positively charged species
C. The movement of a pair of electrons away from a positively charged species
D. The movement of a pair of electrons towards a Lewis base
Markscheme
B
Examiners report
This question generated considerable discussion both in the G2s and on-line. The examiners accept that the question could have been worded better. Candidates are, however, instructed to choose the best answer and this 40% of them did. Interestingly, 36% thought the answer to be A which is clearly wrong.
Which type of reaction occurs when 2-iodo-2-methylpropane, \({\text{C(C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{3}}}{\text{I}}\), reacts with aqueous sodium hydroxide, NaOH(aq)?
A. Addition
B. Free-radical substitution
C. \({{\text{S}}_{\text{N}}}{\text{1}}\)
D. \({{\text{S}}_{\text{N}}}{\text{2}}\)
Markscheme
C
Examiners report
Propene is converted to propanone in a two stage process.
\[{\text{Propene}} \to {\text{X}} \to {\text{Propanone}}\]
What is the formula of compound X?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHBrC}}{{\text{H}}_{\text{3}}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHOHC}}{{\text{H}}_{\text{3}}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
Markscheme
C
Examiners report
Chloroethane, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{Cl}}\), reacts with aqueous sodium hydroxide, NaOH, to form ethanol, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}}\).
Which statement about the mechanism of this reaction is correct?
A. The reaction follows an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism.
B. Homolytic fission of the carbon-chlorine bond occurs in chloroethane.
C. The reaction is unimolecular.
D. The transition state formed is negatively charged.
Markscheme
D
Examiners report
Answers A and B were often chosen as candidates presumably thought the mechanism to be either \({{\text{S}}_{\text{N}}}{\text{1}}\) or free radical.
Which type of halogenoalkane is the substance shown below, and which type of nucleophilic reaction does it undergo with an aqueous sodium hydroxide solution?
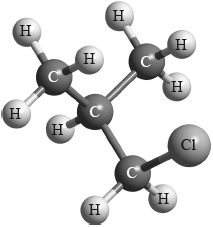
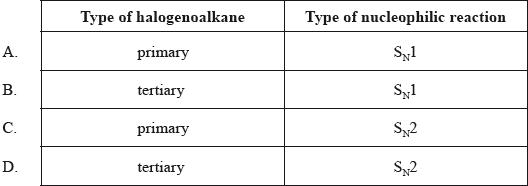
Markscheme
C
Examiners report
What is the type of mechanism and an important feature of the reaction between \({\text{C(C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{3}}}{\text{Br}}\) and aqueous NaOH?
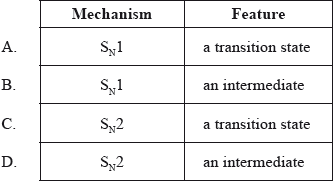
Markscheme
B
Examiners report
There were several comments on this question. One respondent stated that the terms were not covered in their IB textbook. It should be emphasised that it is the guide which defines the syllabus ONLY and NOT any one particular textbook which may be written for the programme itself. Other respondents stated that terms such as transition state and intermediate were not covered in the programme. However, this question relates to AS 10.5.2 and as such it is expected that some universally used terms would be introduced to candidates in explaining both \({{\text{S}}_{\text{N}}}\) mechanisms. The question certainly was very challenging for candidates and only 32.68% of candidates got the correct answer B.
From which monomer is this polymer made?
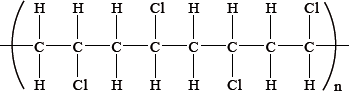
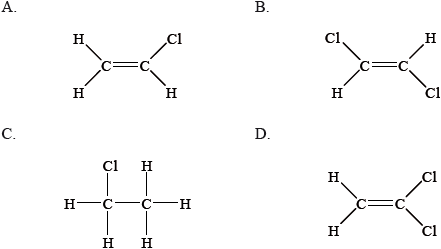
Markscheme
A